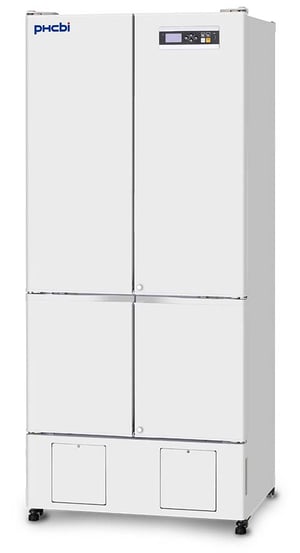
CliniCool© Silver Series 10.5 Cu. Ft. NSF Certified Compact Pharmacy Refrigerator | Solid Door
Item#: LHP-11-SD-PHNSFThese cutting-edge medical refrigerators are certified in accordance with the NSF/ANSI 456 Standard for Vaccine Storage. With this certification, units protect pharmaceuticals at optimal temperatures, preventing waste and allowing for peak delivery. These NSF certified solid door refrigerators utilize microprocessor controllers and feature temperature alarms, remote alarm contacts, and probe access ports with included probes. Units run on natural, hydrocarbon refrigerant for environmental health and energy efficiency.
Includes: Temperature Monitor Device - Complies with The Current CDC Guidelines, 3 Years Certification Of Calibration, “Buffered” Probe In The Product Simulated Solution, Min/Max Memory, °F/°C Switchable, Field Installable, And Visual & Audible Temperature Alarms.
What does NSF certified vaccine storage mean? Click here to learn more
$5,500.00 $4,235.00
- Temperature setpoint range: +1°C to +10°C (Controller settings must remain unaltered to ensure thermal performance compliant with NSF/ANSI 456 Standard for Vaccine Storage requirements)
- Operational environment: Indoor use only, +18°C to +26°C (+65°F to +78°F), <70% RH
- Storage capacity: 10.5 cu. ft. gross volume
- Exterior Dimensions (WxDxH): 23 3/4" x 26" x 59"
- Interior Dimensions (WxDxH): 19 7/8" x 18 3/8" x 50"
- Door: One swing solid door, self-closing, right hinged, non-reversible, magnetic sealed gasket, keyed lock
- Shelves: Seven shelves (six adjustable/one fixed) with guard rail on back
- Mounting and Installation: Low profile roller wheels and leveling legs. Note: 4" of clearance on all sides must be maintained for adequate ventilation
- Interior lighting: Shielded, switched LED lighting, full coverage, balanced spectrum
- Airflow management: Forced Air technology, patent pending
- External probe access: Rear wall port (3/8") dia.
- Insulation: Cabinet is foamed-in-place with EPA compliant high density urethane foam
- Exterior materials: White powder coated steel
- Access control: Pyxis®, Omnicell® and AcuDose RX® compatible
- General warranty: Two (2) years parts and labor warranty, excluding display probe calibration
- Compressor warranty: Five (5) years compressor warranty
- Product Weight: 154 lbs.
- Shipping Weight: 189 lbs.
- Rated Amperage: 2.3 Amps
- Power Plug/Power Cord: NEMA 5-15 plug, 8 to 10 ft typical, conforms to UL471 requirements, Vaccine storage power cord warning label
- Facility Electrical Requirement: 110-120V AC: 15 A (minimum)
- Agency Listing and Certification: Certified with the temperature performance requirements as defined in the NSF/ANSI 456 Standard for Vaccine Storage for all testing scenarios. UL, C-UL, ETL, C-ETL listed and certified to UL471 standard, hydrocarbon refrigerant safety.
- Included Accessories:
- Pharmacy refrigerator/freezer toolkit and temperature logs
- Temperature Monitor Device - Complies with The Current CDC Guidelines, 3 Years Certification Of Calibration, “Buffered” Probe In The Product Simulated Solution, Min/Max Memory, °F/°C Switchable, Field Installable, And Visual & Audible Temperature Alarm.
Performance
- Uniformity¹ (Cabinet air): +/- 1.0°C
- Stability² (Cabinet air): +/- 0.8°C
- Maximum temperature variation (Cabinet air): +/- 1.3°C
- Temperature rise after an after 8 sec door openings: Temperature did not exceed 5.8°C at any probe for all required NSF/ANSI 456 testing protocols
- Recovery after 3 min door opening: All probes recover to under 8°C within 3.5 min.
- Energy consumption: 2.42 KWh/day
- Average heat rejection: 3.27 KWh/day (465 BTU/h)
- Noise pressure level (dBA): 49 or less installed
- Pull down time to nominal operating temp: 25 min
Note: The CDC requires a data logger or temperature monitoring device to consistently record live temperature readings from the inside of a refrigerator or freezer when storing all types of vaccines. Constant monitoring of vaccines at recommended temperatures is essential for maintaining the efficacy of a vaccine. Failing to do so can lead to wasted vaccines or ineffective vaccines being administered to patients. Please follow the CDC requirements for Vaccine Storage & the COVID-19 Vaccination Program found here.
Controller, Configuration, Alarms and Monitoring
- Controller technology: Parametric, microprocessor, LED display with 0.1°C resolution
- Display Technology: NSF/ANSI 456 Standard for Vaccine Storage compliant digital temperature display and alarm module with battery back-up, F/C switchable.
- Temperature setpoint range: +1°C to +10°C (Controller settings must remain unaltered to ensure thermal performance compliant with NSF/ANSI 456 Standard for Vaccine Storage requirements)
- Display probe: Calibrated, stainless steel
- External alarm connection:
- State switching remote alarm contacts.
- Visual and audible indicators.
- Alarms: High / Low temperature, compliant with alarm requirements defined in the NSF/ANSI 456 Standard for Vaccine Storage
- Simulator ballast: Glass bead thermal media
Refrigeration System
- Compressor: Hermetic, high performance
- Refrigerant: EPA SNAP compliant, R600a, Isobutane
- Condenser: Tube and grid construction, fanless
- Evaporator: Plate wall
- Defrost: Cycle optimized, zero energy
LHP-11-SD-PHNSF Datasheet
DownloadLabRepCo CliniCool NSF Certified Refrigerator & Freezer Owners Manual
Download-
Basket Fixed Basket Assembly for 10.5 cu. ft. LabRepCo Silver Series Upright Refrigerators
Item #: LRC-BSKT-BASE-FIXED-10.5$134.00$103.18 -
Custom UPS Battery Backup Systems
Item #: BATTERYBACKUP -
NIST Traceable® High-Accuracy Refrigerator/Freezer Thermometer (1 Probe)
Item #: LABC3-4238$112.30$106.69 -
Basket Slide Assembly for 10.5 cu. ft. LabRepCo Silver Series Upright Refrigerators
Item #: LRC-SLD-BSKT-FULL-10.5$324.00$249.48 -
NIST Traceable® Excursion-Trac™ Refrigerator/Freezer Data Logging Thermometer for Vaccine Storage (1 Probe)
Item #: LABC3-6430-3$171.00$162.45 -
NIST Traceable® Memory-Loc™ Refrigerator/Freezer Data Logging Thermometer for Vaccine Storage (1 Probe)
Item #: LABC3-6440-3$195.00$185.25
Request a Proposal
Related Products
-
Item #: MPR-N450FSH-PA
$8,994.00$5,936.04Item #: MPR-N450FH-PA$9,481.00$6,257.46Item #: MPR-N250FH-PA$6,894.00$4,550.04Item #: MPR-N250FSH-PA$6,641.00$4,383.06Item #: LHP-11-HG-PHNSF$5,500.00$4,235.00Submit a PO
Looking to submit a purchase order rather than buying online? Click on the Submit a PO button to learn more.
Sales & Support
Customer Service
Mon - Fri: 8AM to 5PM ET
101 Witmer Road, Suite 700
Horsham, PA 19044
Contact Sales
Need more information?
Would you like us to get in touch with you? Fill out the form below and we will contact you.